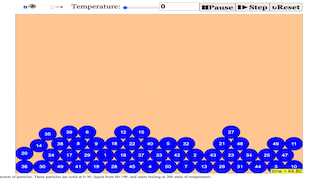
About
This will be a EJSS-based particle physics simulation
Translations
| Code | Language | Translator | Run | |
|---|---|---|---|---|
 |
||||
Credits

 rytan451; lookang
rytan451; lookang
Main Themes:
- Particle-Based Simulation of Matter: Both sources center around a simulation that models the behavior of matter in its solid, liquid, and gaseous states using a particle approach. This aligns with the kinetic model of matter.
- States of Matter: The core concept explored is the distinction between the three common states of matter: solid, liquid, and gas. The simulation aims to visually represent the characteristic properties of each state based on particle arrangement and movement.
- Educational Resource: The simulation is presented as an Open Educational Resource (OER), indicating its intended use in educational settings to facilitate learning about the states of matter and the kinetic model.
- Technology and Collaboration: The sources highlight the use of specific technologies (EJSS, JavaScript, HTML5) and the collaborative nature of the project, crediting "rytan451; lookang" as the authors/creators.
Most Important Ideas and Facts:
- The simulation is a "JavaScript Simulation Applet HTML5" based on the "Kinetic Model". This is explicitly stated in the second source and underscores the underlying scientific principle guiding the simulation's design.
- The simulation is compiled with "EJS 6.1 BETA". The first source mentions this, providing technical detail about the software used to create the simulation.
- The simulation is released under a "license". While the specific license details are not fully present in the excerpt from the first source, the mention of a license indicates its open nature for use, likely related to the OER context. The second source confirms its OER status.
- The simulation is available through "Open Educational Resources / Open Source Physics @ Singapore". The second source details the platform hosting the simulation and its OER nature. It provides information on how to embed the simulation into a webpage via an iframe.
- The simulation aims to visualize the "Phase Change between States of Matter". This is the explicit title of the first source and a core function of the simulation. It implies the simulation can demonstrate transitions between solid, liquid, and gas states.
- The simulation is credited to "rytan451; lookang". Both sources attribute the creation of the simulation to these individuals, highlighting their contribution to the OER community.
Quotes from Original Sources:
- "Phase Change between States of Matter: Solid, Liquid and Gaseous States" - Title of the first source, summarizing the simulation's focus.
- "Using Particles to simulate Three States of Matter in Solid, Liquid and Gaseous State JavaScript Simulation Applet HTML5" - Title of the second source, detailing the method and technology used.
- "Kinetic Model" - Under the "Breadcrumbs" in the second source, indicating the scientific framework.
- "rytan451; lookang" - Credited as authors/creators in both sources.
- "Compiled with EJS 6.1 BETA (201115)" - From the first source, detailing the development environment.
- "Released under a license" - From the first source, indicating the terms of use.
- "Open Educational Resources / Open Source Physics @ Singapore" - The platform hosting the simulation, as indicated in the second source.
In summary, these sources describe an open-source, particle-based simulation created using EJSS, JavaScript, and HTML5, designed to help users understand the three states of matter and the transitions between them based on the kinetic model. It is available as an educational resource through the Open Educational Resources / Open Source Physics @ Singapore platform.
Phase Changes and States of Matter Study Guide
Quiz
- Describe the particle arrangement in a solid.
- How do the particles in a liquid differ in movement compared to particles in a solid?
- What characteristic defines the shape of a gas?
- What is a phase change?
- What happens to the energy of particles when a substance changes from a liquid to a gas?
- Explain why gases can be easily compressed.
- In which state of matter do particles have the strongest attractive forces between them?
- What role do particles play in simulating states of matter according to the source materials?
- What happens to the volume of a liquid when it is heated?
- How is the Open Source Physics @ Singapore resource described in relation to simulating states of matter?
Answer Key
- In a solid, particles are closely packed in a fixed arrangement and vibrate about their fixed positions.
- Particles in a liquid are still closely packed but can move past each other, giving liquids a definite volume but an indefinite shape.
- A gas takes the shape of its container and its volume is also indefinite, expanding to fill the available space.
- A phase change is the transition of a substance from one state of matter (solid, liquid, or gas) to another, typically due to changes in temperature or pressure.
- When a substance changes from a liquid to a gas, the particles gain enough energy to overcome the attractive forces holding them together in the liquid state.
- Gases can be easily compressed because the particles are far apart with significant empty space between them.
- Particles in a solid have the strongest attractive forces between them, holding them in their fixed positions.
- According to the source materials, particles are used to simulate the three states of matter, demonstrating their arrangement and movement.
- When a liquid is heated, its volume typically increases slightly as the particles gain energy and move further apart, though the effect is less dramatic than in gases.
- The Open Source Physics @ Singapore resource is described as using particles to simulate the three states of matter through a JavaScript Simulation Applet HTML5 model.
Essay Questions
- Compare and contrast the arrangement, movement, and intermolecular forces between particles in the solid, liquid, and gaseous states of matter. Provide specific examples for each state.
- Discuss the process of phase changes (melting, freezing, boiling, condensation, sublimation, deposition) in terms of particle behavior and energy transfer.
- Explain how a particle simulation model, like the one described in the source material, can be used to illustrate the concepts of states of matter and phase changes to students. What are the benefits and limitations of using such a simulation?
- Analyze the relationship between temperature, kinetic energy of particles, and the state of matter. How does increasing or decreasing temperature affect the state of a substance?
- Describe the characteristics of gases, focusing on why they have indefinite shape and volume and why they are compressible. Relate these characteristics to the particle model of matter.
Glossary of Key Terms
- Solid: A state of matter where particles are closely packed in a fixed arrangement and vibrate about fixed positions. Solids have a definite shape and volume.
- Liquid: A state of matter where particles are closely packed but can move past each other. Liquids have a definite volume but take the shape of their container.
- Gas: A state of matter where particles are far apart and move randomly and rapidly. Gases have no definite shape or volume and expand to fill their container.
- Phase Change: The physical process where a substance changes from one state of matter to another.
- Particles: The fundamental units of matter (atoms, molecules, or ions) that make up substances.
- Kinetic Model: A model that describes the properties of matter in terms of the motion and arrangement of its constituent particles.
- Simulation: A representation of a real-world process or system, often used for educational or research purposes.
- JavaScript Simulation Applet HTML5: A technical term referring to a type of interactive web application built using JavaScript and HTML5, capable of running simulations.
- Open Educational Resources (OER): Educational materials that are freely available for anyone to use, adapt, and share.
- Open Source Physics: A project dedicated to providing free and open-source software for physics education.
Sample Learning Goals
[text]
For Teachers
[text]
Research
[text]
Video
[text]
Version:
Other Resources
[text]
Frequently Asked Questions
What are the three main states of matter discussed in the sources?
- The sources explicitly mention the three main states of matter as Solid, Liquid, and Gaseous states.
What is a "Phase Change" in the context of these sources?
- The title of one source, "Phase Change between States of Matter: Solid, Liquid and Gaseous States," indicates that a phase change refers to the transition or transformation of matter from one state (solid, liquid, or gas) to another.
What is the purpose of the "Using Particles to simulate Three States of Matter" simulation?
- The purpose of the simulation, as described in the source "Using Particles to simulate Three States of Matter in Solid, Liquid and Gaseous State JavaScript Simulation Applet HTML5," is to use particles to visually represent and simulate the behavior of matter in its solid, liquid, and gaseous states.
What technology is used to create the simulation mentioned in the sources?
- The simulation is created using JavaScript and HTML5, and it is described as an "EJSS-based particle physics simulation." EJSS stands for Easy JavaScript Simulations, which is a toolkit for creating interactive simulations.
Who are the credits attributed to for the materials in these sources?
- The credits for the "Phase Change between States of Matter" excerpts and the "Using Particles to simulate Three States of Matter" simulation are attributed to rytan451 and lookang.
Where can the simulation mentioned in the sources be embedded?
- The source provides an embed code, indicating that the simulation can be embedded within a webpage using an iframe.
What type of educational resource are these materials classified as?
- These materials are described as "Open Educational Resources / Open Source Physics @ Singapore," suggesting they are freely available educational materials related to physics.
Are there sample learning goals available for the simulation?
- Yes, the source mentions that there are "Sample Learning Goals" available, although the actual content of these goals is not included in the provided excerpt.
- Details
- Written by Loo Kang Wee
- Parent Category: 12 Temperature & Ideal Gases
- Category: 01 Kinetic Model
- Hits: 7523