https://youtu.be/ma95EpTQgW8?si=BVbitE50yoqvwY2p
Credits
 ['rytan451', 'lookang']
['rytan451', 'lookang']
1. Introduction:
This briefing document summarizes the key themes and important ideas presented in the provided excerpts related to a JavaScript simulation applet focused on the rates of chemical reactions and the factors that influence them. The primary source is an Open Educational Resource from Open Source Physics @ Singapore, which includes descriptions of the simulation, learning goals, prerequisite knowledge, potential student misconceptions, and links to supplementary materials.
2. Main Themes and Important Ideas:
The central theme of these resources is the exploration and understanding of the factors affecting the rate of chemical reactions. The simulation applet serves as an interactive tool for students to visualize and manipulate these factors, observing their impact on the reaction speed. The key concepts highlighted across the sources are:
- Collision Theory: Reactions occur when reacting species collide with sufficient energy (activation energy). This is explicitly stated as a "Big idea/Concept": "Reactions happen only when the reacting species collide with each other with sufficient energy (aka activation energy)."
- Environmental Factors: Reaction rates are dependent on environmental conditions. The simulation specifically focuses on:
- Concentration of Reactant 1 (Blue): The amount of reactant present in a given volume.
- Temperature: The degree of hotness or coldness, related to the kinetic energy of particles.
- Amount and Surface Area of Reactant 2 (Red): The quantity and exposed surface of the other reactant.
- Measuring Reaction Rates: Reaction rates can be determined by observing changes in the concentrations of reactants or products over time. This is mentioned within the "Big idea/Concept": "Reaction rates are dependent on environmental factors (e.g. temperature, and concentration). They can be determined by measuring changes in concentrations of reactants or products over time."
- Effective vs. Ineffective Collisions: Not all collisions lead to a reaction. Collisions must have sufficient energy and proper orientation to be effective. This is addressed as a "Learning Issue to be addressed": "Students have the misconception that all collisions are effective collisions which leads to a reaction."
3. Details from the Resources:
3.1 Simulation Description and Access:
- The resource describes a "JavaScript Simulation Applet HTML5" designed to demonstrate how "various starting conditions influence how quickly a reaction proceeds."
- The applet allows users to "Watch the particles interact in real time," "Configure starting and boundary conditions," "See how many of each type of particle there are," "Change the surface area of a reactant," and "Watch until it's all reacted."
- The simulation can be embedded in a webpage using an provided iframe code.
- Direct access to the simulation is provided via a link: https://sg.iwant2study.org/ospsg/index.php/interactive-resources/chemistry/03-chemistry-of-reactions/03-chemical-reactions/1144-rate-of-reaction
3.2 Learning Goals and Level:
- The simulation is intended for Sec 4 Chemistry O-Level Chemistry / O-Level Science (Chemistry) students.
- It aligns with the topic of "Rate of Reaction (Factors affecting Rate of Reaction)."
3.3 Prerequisite Knowledge:
Students are expected to have prior understanding of:
- Particulate Nature of Matter
- Concepts such as temperature in relation to kinetic energy
- Acid reactions and concentration of a solution
3.4 Addressing Student Misconceptions:
The resource explicitly outlines common student misconceptions related to the factors affecting reaction rates:
- Effectiveness of Collisions: Students incorrectly believe all collisions result in a reaction. The simulation likely aims to visually differentiate between successful and unsuccessful collisions based on energy.
- Temperature and Energy: When discussing temperature, students often focus solely on the increased speed of particles, neglecting the crucial aspect of whether more particles possess sufficient activation energy. The resource highlights the need to mention the "energetic aspect (i.e. whether more/fewer particles have energy equal to or more than activation energy)."
- Surface Area and Particle Size: Students may wrongly equate larger particle size with larger surface area. The simulation likely allows for the manipulation of surface area independent of the overall amount of the reactant.
- Concentration: Students tend to explain concentration using "more/fewer particles" without specifying "per unit volume." This emphasizes the importance of defining concentration as the amount of substance in a given space.
- Frequency vs. Number of Products: Students may confuse the rate or frequency of collisions with the total number of products formed. The simulation can help differentiate between how fast the reaction occurs and how much product is ultimately produced.
3.5 Supplementary Resources:
The resource provides a valuable collection of links to:
- Videos: Demonstrating the effect of temperature and concentration on reaction rates, as well as the effect of surface area and particle size. Examples include "Effect of Temperature on Speed of Reaction" and "How does concentration affect rate of reaction?".
- Interactive Simulations: From CK12 and PhET, covering related concepts such as states of matter, phases of matter, and gas behavior (including collisions and pressure). The PhET simulation on gases is suggested for exploring the relationship between pressure and the rate of reaction through collision frequency.
3.6 For Teachers:
A link to a "SLS CG lesson by Daryl" is provided, which offers an "educational demonstration of factors affecting the rate of reaction" using a "realistic physics model." This suggests that teachers have access to curated lesson plans and activities utilizing the simulation.
4. Quotes from Original Sources:
- Big idea/Concept: "Reactions happen only when the reacting species collide with each other with sufficient energy (aka activation energy)."
- Big idea/Concept: "Reaction rates are dependent on environmental factors (e.g. temperature, and concentration). They can be determined by measuring changes in concentrations of reactants or products over time."
- Learning Issue to be addressed: "Students have the misconception that all collisions are effective collisions which leads to a reaction."
- Learning Issue to be addressed: "For the factor of temperature, students tend to focus on the rate of reacting particles, forgetting to mention the energetic aspect (i.e. whether more/fewer particles have energy equal to or more than activation energy)"
- Learning Issue to be addressed: "Students have the misconception that larger particle size = larger surface area"
- Learning Issue to be addressed: "Students tend to explain the factor of concentration using “more/fewer particles” rather than “more/fewer particles per unit volume”"
5. Conclusion:
The provided resources offer a comprehensive approach to teaching and learning about the factors affecting the rate of chemical reactions. The JavaScript simulation applet provides a valuable interactive platform for students to visualize abstract concepts and test hypotheses. The explicit identification of common misconceptions and the provision of supplementary materials, including videos and related simulations, further enhance the educational value of this resource. This collection of materials is well-suited for O-Level Chemistry students and provides teachers with effective tools for engaging students with this fundamental topic in chemistry.
Study Guide: Rates of Reactions
Overview
This study guide is designed to help you review the factors affecting the rate of chemical reactions, based on the provided resources. The resources include text excerpts outlining learning goals and addressing common misconceptions, links to an interactive simulation, and relevant videos explaining the concepts. The focus is on understanding how concentration of reactants, temperature, and the amount and surface area of another reactant influence reaction rates.
Key Concepts
- Collision Theory: Reactions occur when reactant particles collide with sufficient energy (activation energy) and the correct orientation.
- Rate of Reaction: The speed at which a chemical reaction proceeds, typically measured by the change in concentration of reactants or products over time.
- Concentration of Reactants: The amount of a reactant present in a given volume.
- Temperature: A measure of the average kinetic energy of particles.
- Activation Energy: The minimum energy required for a collision between reactant particles to result in a chemical reaction.
- Surface Area: The total area exposed by a solid substance.
- Amount of Reactant: The quantity or moles of a reactant present.
- Effective Collisions: Collisions between reactant particles that result in a chemical reaction.
Review Questions
Consider the following questions as you review the sources:
- What are the fundamental requirements for a chemical reaction to occur according to collision theory?
- How does increasing the concentration of a reactant affect the frequency of collisions between reactant particles? How does this relate to the rate of reaction?
- Explain how an increase in temperature affects both the kinetic energy of reactant particles and the number of particles possessing sufficient activation energy. How do these changes influence the rate of reaction?
- How does increasing the surface area of a solid reactant affect the rate of reaction? Why does this occur at the particle level?
- What is the difference between the frequency of collisions and the number of product molecules formed?
- What are some common misconceptions students have about the factors affecting reaction rates?
- How can the provided simulation help visualize the effect of different factors on the rate of reaction?
- What aspects of the particle behavior in the simulation demonstrate the principles of collision theory?
- How do the provided videos illustrate the impact of concentration, temperature, and surface area on reaction rates?
- What prior knowledge is recommended for a thorough understanding of this topic?
Quiz: Short Answer Questions
Answer the following questions in 2-3 sentences each.
- According to collision theory, what two conditions must be met for a collision between reactant particles to be effective and lead to a reaction?
- Explain how increasing the concentration of a reactant leads to an increase in the rate of a chemical reaction at the molecular level.
- How does an increase in temperature affect the kinetic energy of reactant particles, and what is the significance of this change in relation to activation energy?
- Why does increasing the surface area of a solid reactant often lead to a faster rate of reaction? Provide an explanation based on particle interactions.
- Describe a common misconception students have regarding the effect of temperature on reaction rates, as highlighted in the learning issues.
- Explain why simply having "more particles" of a reactant doesn't fully explain the effect of concentration on reaction rate; what is a more precise way to describe it?
- What does the term "activation energy" refer to in the context of chemical reactions, and why is it important for a reaction to occur?
- In the provided resources, what is the core idea or concept linking collisions of reacting species to the occurrence of a reaction?
- Explain the difference between the frequency of collisions between reactant particles and the number of product molecules that are formed.
- How can the interactive simulation referenced in the sources be a valuable tool for understanding the factors affecting reaction rates?
Quiz: Answer Key
- For a collision to be effective, reactant particles must collide with sufficient kinetic energy, equal to or greater than the activation energy, and they must also collide with the correct orientation for bond breaking and formation to occur.
- Increasing the concentration of a reactant means there are more reactant particles in a given volume. This leads to a higher frequency of collisions between reactant particles, increasing the likelihood of effective collisions and thus a faster reaction rate.
- An increase in temperature increases the average kinetic energy of all reactant particles, causing them to move faster and collide more frequently. Importantly, a higher temperature also increases the proportion of particles possessing kinetic energy equal to or greater than the activation energy, leading to more effective collisions.
- Increasing the surface area of a solid reactant exposes more of its particles to the other reactants. This results in a greater number of potential collision sites and a higher frequency of collisions between the reactants, thereby increasing the rate of reaction.
- A common misconception is that increasing temperature only increases the speed of reacting particles, while neglecting to mention that it also increases the number of particles with sufficient energy to overcome the activation energy barrier.
- The effect of concentration should be explained using the idea of "more or fewer particles per unit volume," as this directly influences the frequency of collisions. Simply stating "more particles" doesn't account for the volume and thus the likelihood of collisions.
- Activation energy is the minimum amount of energy that colliding reactant particles must possess for a chemical reaction to occur. It is the energy barrier that must be overcome for the reaction to proceed by allowing for bond breaking in reactants and bond formation in products.
- The core idea is that chemical reactions at the molecular level are the result of collisions between reacting species, and these collisions must be sufficiently energetic to initiate the transformation of reactants into products.
- The frequency of collisions refers to how often reactant particles bump into each other, regardless of whether these collisions lead to a reaction. The number of product molecules formed depends on the number of effective collisions, which are those with sufficient energy and correct orientation.
- The interactive simulation allows for a visual representation of particle collisions and how factors like concentration and temperature affect the frequency and energy of these collisions, providing a concrete way to understand abstract concepts related to reaction rates.
Essay Format Questions
Consider the following questions for a more in-depth exploration of the topic. Develop well-structured essays with clear introductions, body paragraphs supported by evidence from the sources, and concise conclusions.
- Discuss the key principles of collision theory and explain how the concentration of a reactant influences the rate of a chemical reaction based on these principles. Refer to the provided resources to support your explanation, including potential student misconceptions.
- Explain in detail the effect of temperature on the rate of a chemical reaction, emphasizing the role of kinetic energy and activation energy. How does the provided information address common misunderstandings about this relationship?
- Analyze the impact of the surface area of a solid reactant on the rate of reaction. Using the concept of particle interactions and collision theory, elaborate on why an increased surface area typically leads to a faster reaction.
- Critically evaluate the learning goals and identified misconceptions related to the factors affecting reaction rates, as outlined in the provided resources. How can interactive simulations and visual aids help address these misconceptions effectively?
- Synthesize the information from the text excerpts, the description of the interactive simulation, and the video titles to provide a comprehensive overview of the factors that influence the rate of chemical reactions. Discuss how these different resources contribute to a deeper understanding of the topic.
Glossary of Key Terms
- Activation Energy: The minimum energy that reactant molecules must possess in order for a chemical reaction to occur upon collision.
- Collision Theory: A theory stating that chemical reactions occur when reactant molecules collide with sufficient kinetic energy and proper orientation.
- Concentration: The amount of a substance present in a given volume, often expressed in moles per liter (molarity).
- Effective Collision: A collision between reactant molecules that results in a chemical reaction, occurring when the molecules collide with sufficient energy and correct orientation.
- Kinetic Energy: The energy possessed by an object due to its motion. In chemistry, it refers to the energy of movement of atoms and molecules.
- Rate of Reaction: The speed at which a chemical reaction takes place, typically measured by the change in concentration of a reactant or product over time.
- Surface Area: The total area of the surface of a material. For solids, a larger surface area exposed to other reactants can increase the rate of reaction.
- Temperature: A measure of the average kinetic energy of the particles in a substance. Higher temperature indicates higher average kinetic energy.
Sample Learning Goals
Overview
|
Topic: |
Rate of Reaction (Factors affecting Rate of Reaction) |
|
Big idea/ Concept |
|
|
Profile/ Level |
|
|
Prerequisite Knowledge |
|
|
Learning Issue to be addressed |
|
For Teachers

Configure starting and boundary conditions
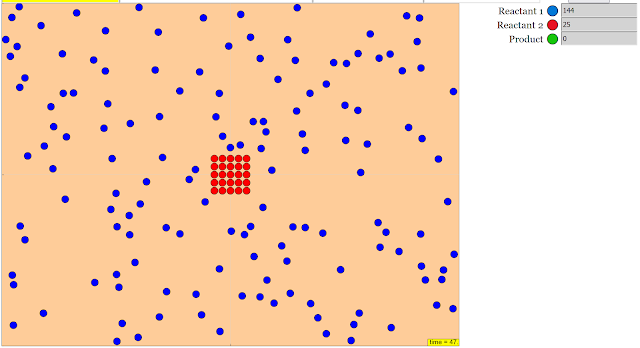
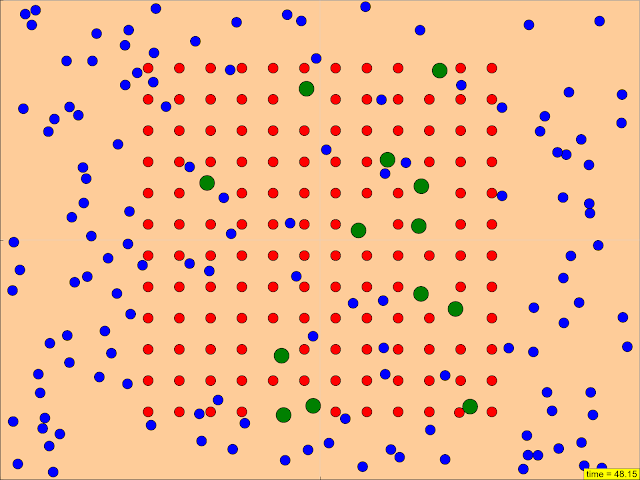
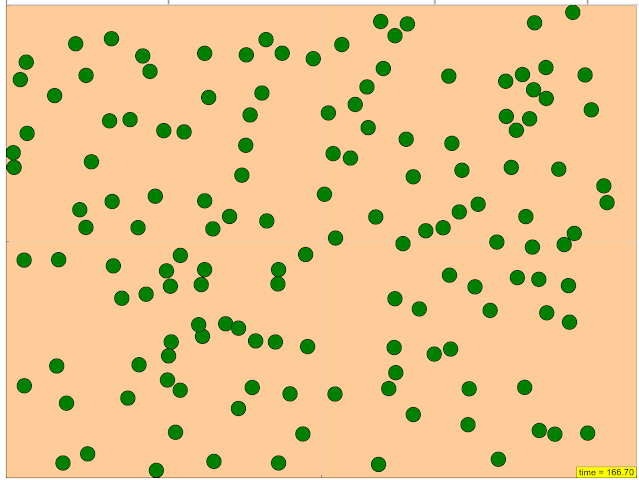
Research
[text]
Video
https://www.youtube.com/watch?v=oeM6hKm6Td4&t=2s Effect of Temperature on Speed of Reaction by ETDtogo
https://www.youtube.com/watch?v=jDmxbFYvQgo How does concentration affect rate of reaction? by ChemJungle
https://www.youtube.com/watch?v=kGJsDDjwP1Y How do Temperature and Concentration Affect the Rate of Reaction? by Chemistry Breakdown
https://www.youtube.com/watch?v=BWN8xVuzuFI The effect of surface area and particle size on the rate of a chemical reaction. by Michael Kavanagh
https://www.youtube.com/watch?v=lukSSS9Hfaw How does surface area affect rate of reaction? by ChemJungle
Version:
Other Resources
https://interactives.ck12.org/simulations/embed.html?embeded=true&interactive=states-of-matter&subject=chemistry&lang=en&assignment=true show the arrangement and movement of particles in the different states of matter are due to the varying strength of the forces of attraction between the particles.
https://interactives.ck12.org/simulations/embed.html?embeded=true&interactive=phases-of-matter&subject=chemistry&lang=en&assignment=true show how solid melts to become a liquid in terms of kinetic particle theory and energy changes!
https://phet.colorado.edu/sims/html/gases-intro/latest/gases-intro_en.html Instructions:
Step 1: Select "Intro"
Step 2: Tick "Collision Counter" tab
Step 3: Expand "Particles" tab by clicking on the "+" icon
Step 4: Add some "Light" (red coloured) particles to the vessel
Step 5: Add in more "Light" particles and observe what happens to:
a. Pressure reading for the vessel
b. Number of Collisions
(remember to start the wall collision counter after you have added the particles and wait for the reading to be taken)
Step 6: Based on your observations from Step 5, go to the "Connect" column below and key in how pressure affects the rate of reaction.
Frequently Asked Questions: Factors Affecting Rates of Reactions
1. What are the fundamental conditions required for a chemical reaction to occur according to the provided resources? The resources emphasize that a reaction happens only when reacting species collide with each other. However, not all collisions result in a reaction. These collisions must occur with sufficient energy, which is also known as the activation energy, for the reaction to proceed.
2. How does the concentration of a reactant influence the rate of a chemical reaction, and what common misconception should students avoid? An increase in the concentration of a reactant generally leads to a faster rate of reaction. This is because a higher concentration means there are more reactant particles per unit volume. Consequently, the frequency of collisions between reactant particles increases, leading to more effective collisions and thus a higher rate of reaction. A common misconception students have is explaining concentration effects simply by saying "more particles," without specifying "more particles per unit volume," which is the crucial aspect.
3. What is the role of temperature in influencing the rate of a chemical reaction, and what is a key aspect students should remember beyond just the speed of particles? Temperature has a significant impact on the rate of reaction. Increasing the temperature causes the reactant particles to move faster and collide more frequently. More importantly, a higher temperature increases the kinetic energy of the particles, meaning a larger proportion of collisions will have energy equal to or greater than the activation energy. Students often focus solely on the increased speed of particles but should also explicitly mention that a higher temperature leads to more particles possessing sufficient energy to overcome the activation energy barrier.
4. How do the amount and surface area of a solid reactant affect the rate of a reaction, and what misconception exists regarding particle size and surface area? The amount of a solid reactant can indirectly affect the rate, especially if it influences the surface area available for reaction. However, the surface area of a solid reactant has a direct impact. A larger surface area (for the same amount of reactant) means more contact points are available for collisions with other reactants, leading to a higher rate of reaction. A common misconception is that larger particle size equates to larger surface area, which is incorrect. Smaller particles have a larger overall surface area compared to a single large particle of the same total volume.
5. What is the difference between collision frequency and effective collisions in the context of reaction rates? Collision frequency refers to the total number of collisions between reactant particles per unit time. However, not all collisions lead to a reaction. For a collision to be effective, the colliding particles must possess sufficient kinetic energy (equal to or greater than the activation energy) and collide with the correct orientation. Only effective collisions result in the breaking of existing bonds and the formation of new ones, thus leading to product formation and contributing to the rate of reaction.
6. What are some practical ways to investigate the factors affecting the rate of reaction, as suggested by the resources? The resources mention a JavaScript simulation applet that allows users to configure starting conditions and observe how various factors like concentration, temperature, and surface area influence the rate at which a reaction proceeds. Additionally, the included videos demonstrate the effects of temperature and concentration on reaction rates visually through experiments. These simulations and demonstrations provide interactive and visual ways to understand these concepts.
7. Why is it important to understand the concept of activation energy when discussing reaction rates? Activation energy is the minimum amount of energy that colliding reactant particles must possess for a reaction to occur. Without sufficient energy to overcome this barrier, collisions will be unsuccessful, and no products will be formed. Understanding activation energy is crucial for explaining why factors like temperature influence reaction rates so significantly, as higher temperatures lead to a greater number of particles having enough energy to exceed the activation energy threshold.
8. What are some of the learning issues or misconceptions students commonly have regarding factors affecting reaction rates, as highlighted in the resources? The resources explicitly mention several common misconceptions:
- Believing that all collisions between reactant particles lead to a reaction.
- When discussing temperature, focusing only on the increased speed of particles and neglecting the energetic aspect (i.e., whether more particles reach activation energy).
- Thinking that larger particle size means larger surface area.
- Explaining concentration effects using just "more/fewer particles" instead of "more/fewer particles per unit volume."
- Confusing the frequency of collisions with the number of products formed.
- Details
- Written by Loo Kang Wee
- Parent Category: 03 Chemistry of Reactions
- Category: 03 Chemical Reactions
- Hits: 18967


.png
)






